Our group develops mathematical and computational models in cell biophysics. Some of our focus areas include:
• Cell motion: how cell motion is driven by assembly and turnover of dynamic actin networks.
• Actin dynamics: kinetics and thermodynamics of actin polymerization and turnover.
• Biopolymer networks: organization, mechanics and image analysis.
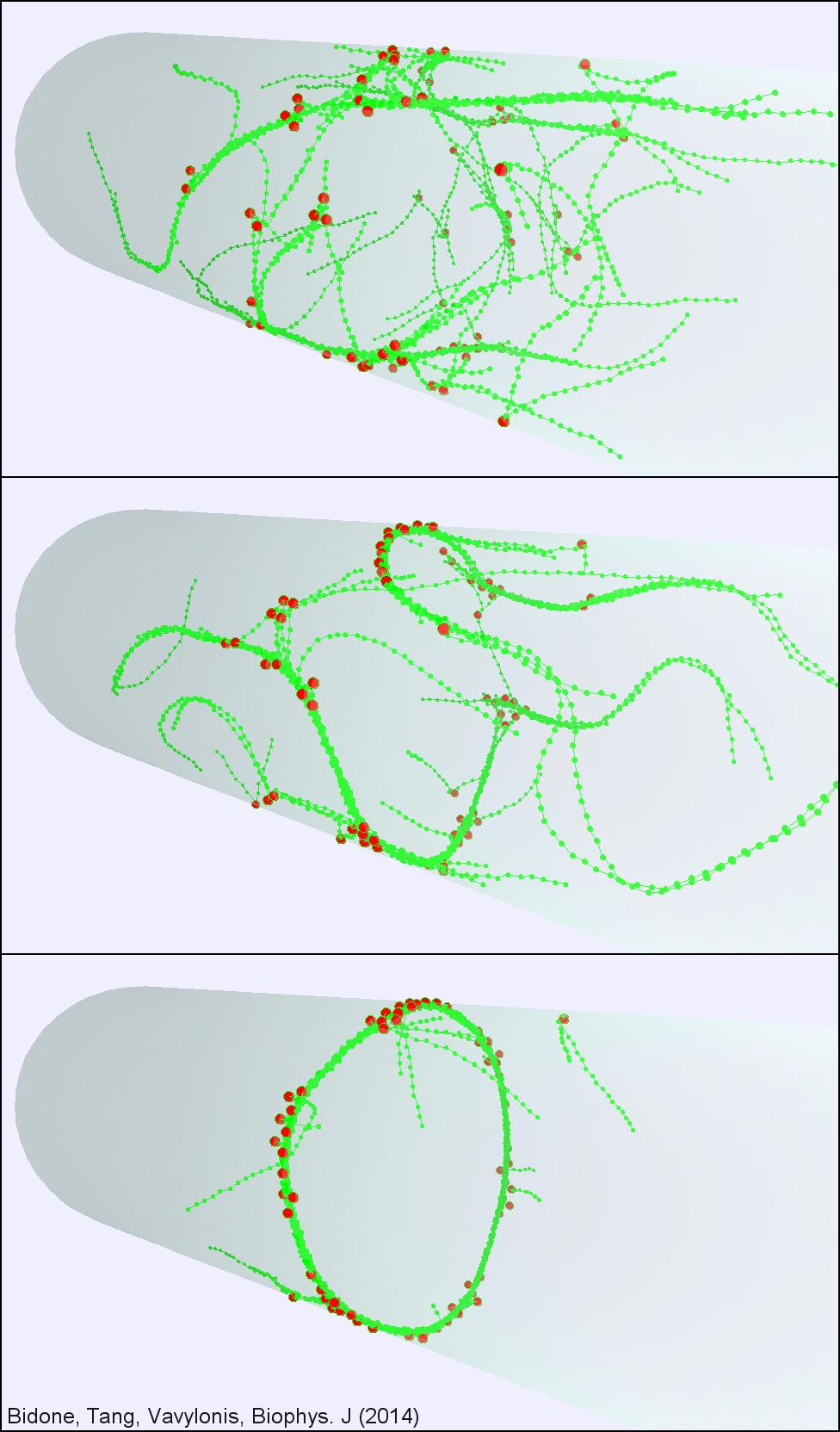
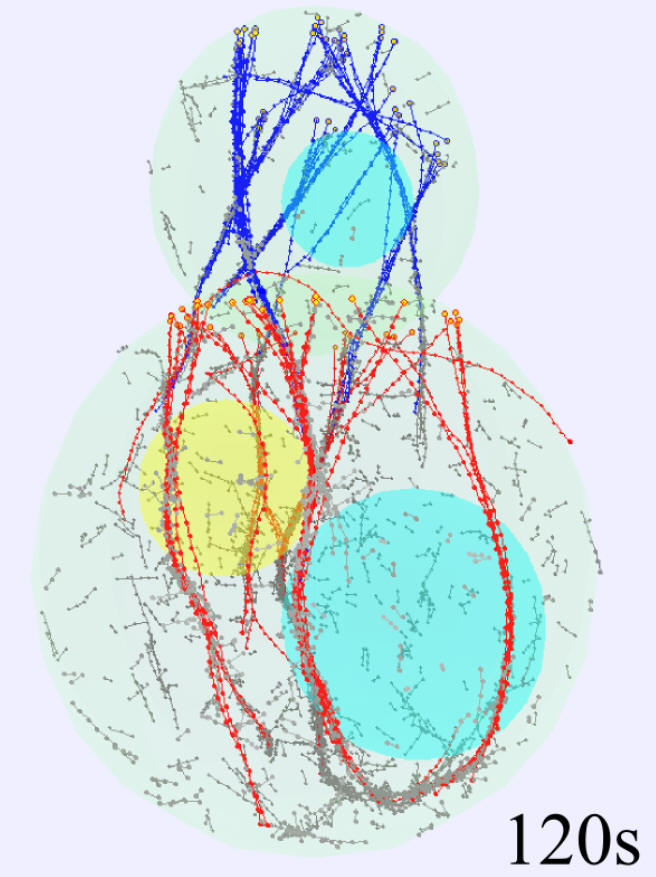
Cytokinesis is the final step in cell division when a dividing cell physically separates into two. During cytokinesis, animals and fungi assemble a contractile ring containing actin filaments and the motor protein myosin to separate into two daughter cells. Despite huge progress in identifying most major protein components, many aspects of the physical mechanism of contractile ring assembly and constriction remain unclear. To better understand this process, we are developing models of ring self-organization and constriction, in collaboration with experimentalists.
In fission yeast, membrane-bound nodes containing myosin are broadly distributed around the cell equator. They assemble into a contractile ring through stochastic motions, after a meshwork of dynamic actin filaments appears. Analysis of node motions and numerical simulations supported a "search, capture, pull and release" mechanism whereby transient connections are established when myosins in one node capture and exert force on actin filaments growing from other nodes.
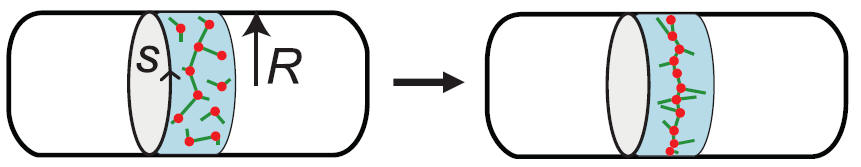
References
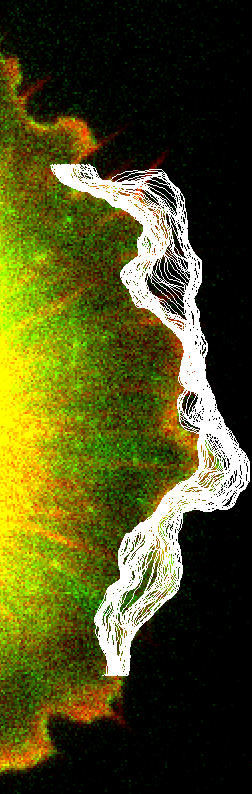
We work to model how actin filament polymerization and depolymerization provides a mechanism for cellular movement and change of shape. We interested on the mechanisms of local and global actin distribution across the cell by diffusion and reaction. We also model the lamellipodium at the leading edge of motile cells, which is a dynamic structure consisting of a dense network of branched actin filaments.
For this work we collaborate with the lab of Naoki Watanabe (Kyoto University), combining modeling, image analysis, and single molecule fluorescence microscopy to quantify the role of diffuse actin species and their gradients in actin reorganization at the cell leading edge.
Our work highlighted that a crucial aspect to the lamellipodium remodeling process is the diffusive motion of the disassembling pieces of the network, the result of actin filament severing. We further developed experimental and computational methods to study the cycle of lamellipodial protrusion and retraction. We found that the Arp2/3 complex accumulates in bursts along the leading edge, in agreement with our mathematical model of protrusion as an excitable system.
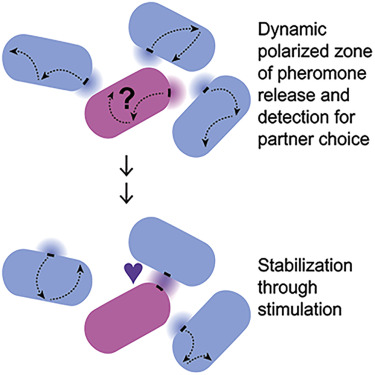
We are interested in understanding how cells establish monopolar and bipolar growth patterns using membrane proteins and the cytoskeleton, and how these patterns contribute to cell shape.
We studied the kinetics of polarity protein Cdc42 that exhibits anti-correlated oscillations at cell tips (in collaboration with the lab of Fulvia Verde, University of Miami). In collaboration with the group of Sophie Martin (University of Lausanne) we study how cells polarize toward their mating partners during fission yeast mating. We have modeled the exploratory search for a partner as an optimization strategy. We also modeled the molecular mechanisms driving this exploration based on the local activation of Ras1.
Our group has developed models of how formin-mediated actin cables organized by polymerization at cell tips in fission and budding yeast.
References
Click here to read about our model of Cdc42 oscillations and how they contribute to the initiation of bipolar growth.
A graphical Java simulation from our first model of actin cable turnover is available here.
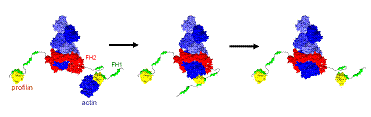
The fast and reversible polymerization of actin proteins into long filaments consisting of hundreds of subunits is the driving force for many types of cell motions. Actin filament growth proceeds via polymerization, depolymerization, ATP hydrolysis and release of phosphate.
We are interested in understanding how the free energy of ATP hydrolysis is used by cells to power constant turnover of actin filaments. We also develop coarse-grained models describing how large protein complexes, such as formins, regulate and accelerate polymerization. Prior work provided a thermodynamically self-consistent model of pure actin filament treadmilling, explaining the origin of the difference in the critical concentration between the barbed and pointed ends. We further modeled how profilin cooperates with formins to accelerate actin filament polymerization by transfer of profilin-actin to the barbed end.


References
How semiflexible filaments organize into bundles and networks with the help of cross-linking and motor proteins is of crucial importance to the mechanics of biological and bioinspired functional materials. Our group develops computational models that account for the discrete nature of filament interactions. We have recently modeled the minimal requirements for ring bundle formation under confinement.
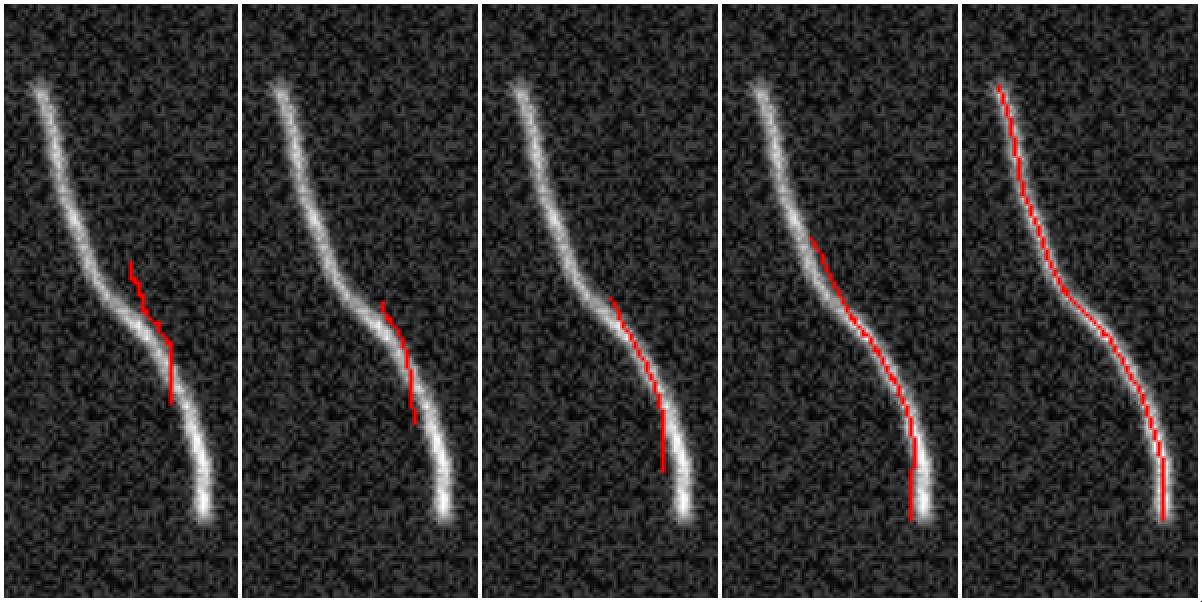
SOAX and TSOAX software extract and track the centerlines and junctions of filamentous networks in 2D/3D images. The underlying method of SOAX and TSOAX uses multiple Stretching Open Active Contours (SOACs) that are automatically initialized at image intensity ridges and then stretch along the centerlines of filaments in the network. Working with the group of D. Cosgrove (PSU), we have applied these methods to quantify the mechanical deformation of plant cell walls imaged by AFM.
Speckle TrackerJ, an ImageJ tool for analyzing trajectories of point-like particles in fluorescence microscopy time-lapse movies. Manual and computer-assisted techniques allow robust tracking of speckles in different situations.
JFilament, is an ImageJ plugin for segmentation, tracking, and visualization of individual fibers. The program uses open active contours to quantify biopolymers, typically imaged by fluorescence microscopy.
A set of ImageJ plugins (LEAP) we developed can be used to quantify cell shape and intensity near the cell edge.
References