CCUS - Carbon Capture, Utilization, and Sequestration
Project 1. Carbon mineralization
Collaborating with Steve Peters (EES), Carlos Romero and Sudhakar
Neti (Lehigh
Energy Resarch Center), Muhannad Suleiman, Paolo Bocchini,
Clay Naito (CEE),
Jonas Baltrusaitis (ChemE),
Karen Beck-Pooley (PolySci), and Alberto Lamadrid (Econ).
Once CO2 is captured and concentrated it needs
to be safely sequestered in geologic reservoirs over geologic time
scales. The traditional way of doing this is by injection
into deep saline aquifers or in permeable, former hydrocarbon
reservoirs; however, there are geographic and geologic barriers to
doing this in eastern Pennsylvania. Here we will investigate
a relatively novel solution consisting of direct back-to-rock CO2
mineralization, using Ca- and Mg-rich waste rock from the
aggregate mining industry. This sequestration option, which takes
advantage of the natural silicate weathering cycle, has the
potential to remove MtCO2/yr from the atmosphere, a
flux that scales well with the current emissions of the
Pennsylvania cement industry. Although Pennsylvania has the
correct rock mineralogy and distribution to justify our enthusiasm
(Fig. 1a), significant knowledge gaps in reaction rates, and
comparative texture and mineralogy of the waste stream
mineralizing the carbon remain. Research projects need to
specifically target the reaction rate problem, focusing on the
grain size (reaction surface area) and mineralogy of the aggregate
waste stream, with an eye on favorable energy conversion processes
that may lead to desirable by-products (Fig. 1b).
We
have reason to be optimistic that diabase and serpentinite waste
generated by the aggregate industry in eastern Pennsylvania is an
excellent material for mineralizing CO2. The
chemistry involves Mg and Ca-rich silicate minerals.
Consider the silicate mineral diopside, which occurs in
diabase aggregate waste at ~30 wt%, to illustrate the process:
MgCaSi2O6 + 2CO2 +
4H2O = MgCO3 (solid)+ CaCO3
(solid)+ 2H4SiO4 (aqueous)
(3)
For every 2 moles of CO2 (44 g/mole; 88 g), the
dissolution of diopside consumes 1 mole of pyroxene (216
g/mole). That is one needs 216 g of rock waste (assuming it
was pure diopside) to neutralize 88 g of CO2, or ~2.5 g
of rock for every 1 g of CO2. For example, the
Pennsylvania cement industry is emitting ~1.8 MtCO2/yr,
which translates to ~5,000,000 m3 of rock at 30 wt%,
diopside to effectively mineralize all of this carbon.
However, diopside is paired with other silicate minerals like
labradorite feldspar, which also dissolves congruently and
consumes CO2, so the necessary rock waste volumes would
be less. Furthermore, even considering the modest known reaction
rates of ~10-11 moles/m2/s, the dissolution
of the Mg- or Ca-rich silicate proceeds over time frames of years
to tens of years. The resulting bicarbonate ion remains in
the Earth surface system for decades to centuries, and the final
natural mineralization takes place over centuries. All of
these are time frames commensurate with what is necessary to
remove anthropogenic CO2 from the atmosphere.
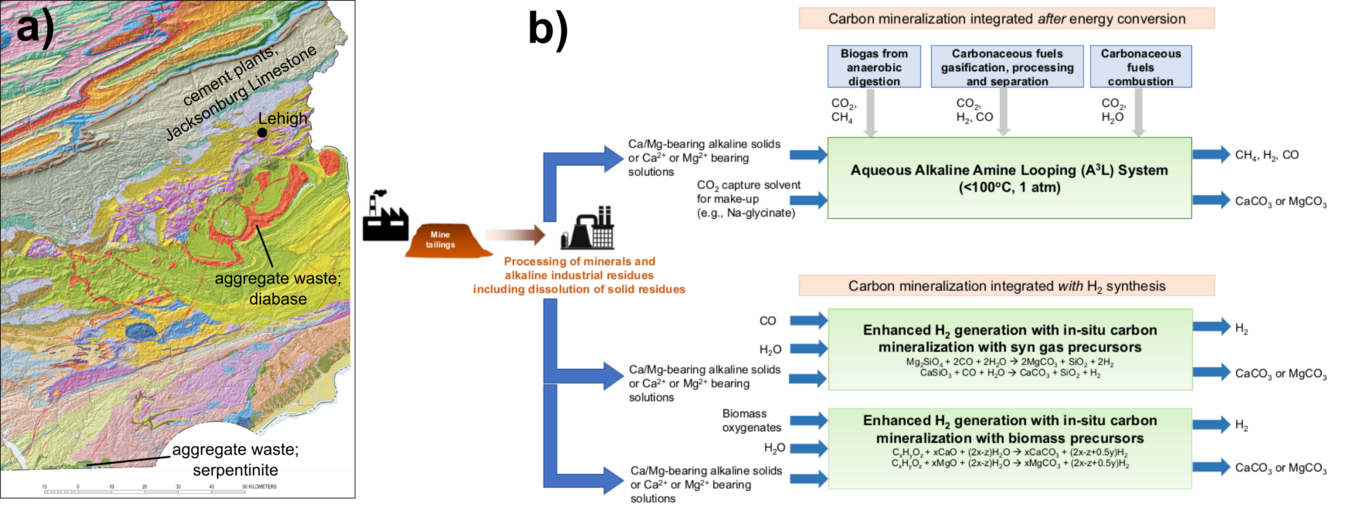
Figure
1. (a) Geologic map of east-central and southeastern PA
showing the close geographic distribution of the cement
industry to the north of Lehigh, and the location of the rocks
south of Lehigh where aggregate waste capable of mineralizing
the CO2 are located. (b) Summary diagram
showing the production of favorable by-products possible in
the CO2 mineralization process from: Gadikota, G.,
2021, Carbon mineralization pathways for carbon capture,
storage, and utilization: Nature Communications Chemistry,
4-23, doi.org/10.1038/s42004-021-00461-x.
Project 2. Carbon sequestration and low-enthalpy geothermal energy.
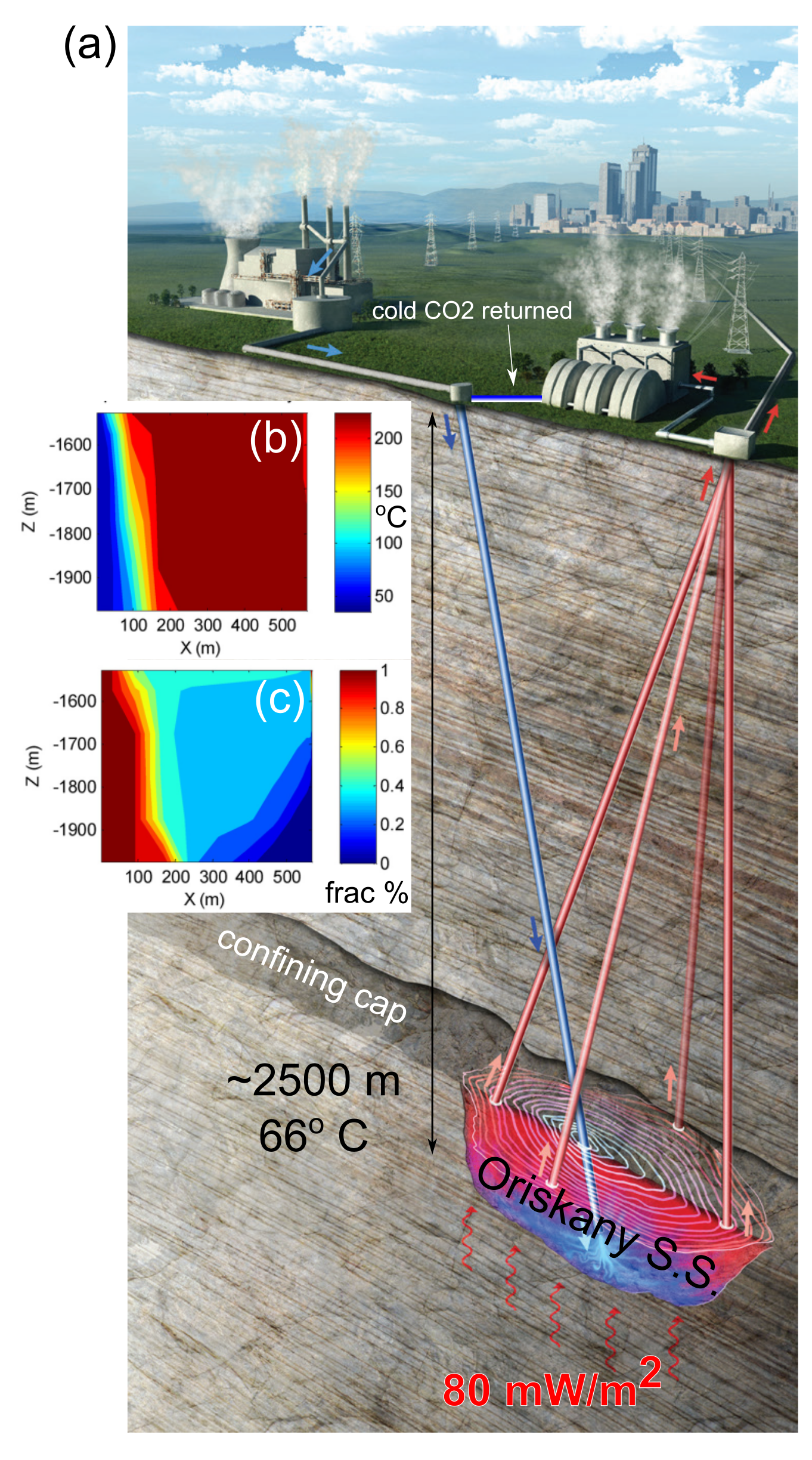
We are researching power generation and/or district heating using a novel super-critical CO2 (sCO2) injection technology that leverages an existing network of CO2 sources and deep wells to harvest energy from a relatively untapped geothermal energy resource proximal to major population centers with growing energy needs (Fig. 1). Unlike traditional geothermal projects that target shallow, hot, dry rock, typically using water or steam, and involve the drilling of new wells, the novelty in our concept emerges from the nexus of: (1) utilization of sCO2 injection to extract large quantities of low-exergy heat, (2) re-purposing existing, suitable hydrocarbon wells, (3) mining heat from an anomalously warm part of the Appalachian Basin (Stutz et al., 2015; Fig. 2), and (4) realizing an associated benefit of partial CO2 storage. This multi-phase project aims to generate power from a comparatively low-temperature geothermal source, demonstrating its potential when it is scaled and applied to warmer sources elsewhere in the country.

Fig. 1. (a) Illustration of our concept for extracting geothermal heat using sCO2 from a conventional natural gas reservoir in the Appalachian Basin, in this case shown as the Oriskany Sandstone, which is already penetrated by existing wells. Observation-based modeling results from the Lehigh group showing (b) geothermal reservoir temperature and (c) percent sCO2 saturation after 5 years for comparable depths and distances between the injection and production wells proposed for this project (modified from Pan et al., 2018).
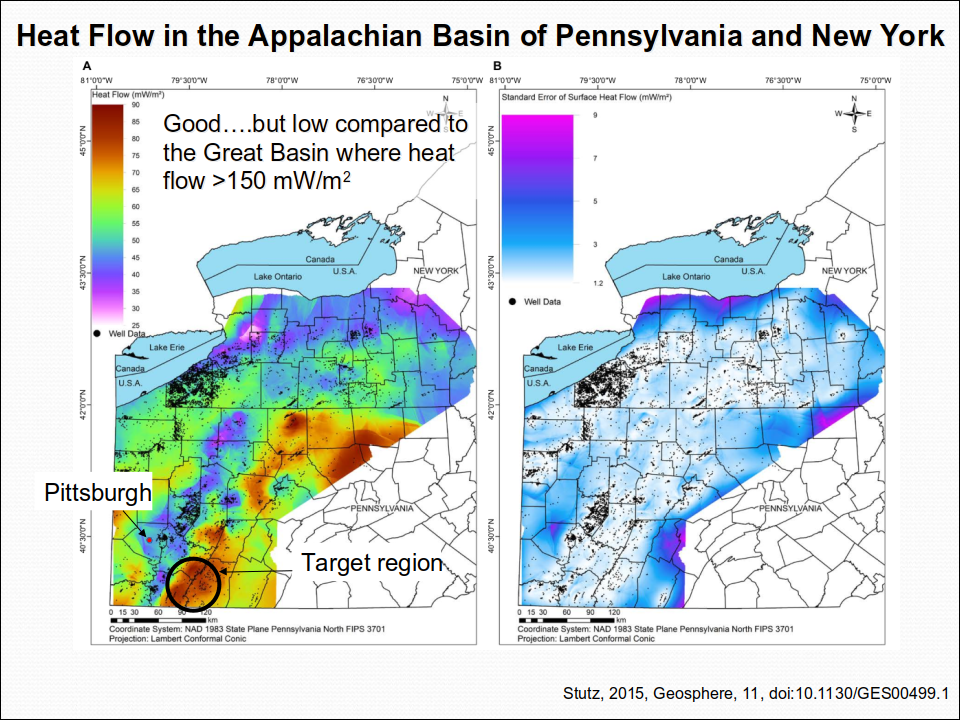
Figure 2. A Heat flow and B standard error in heat flow calculation for the Appalachian Basin in Pennsylvania and New York. (Stutz et al., 2015, Geosphere, 11, doi:10.1130/GES00499.1).